
Chemistry, 18.02.2021 19:30, savannahvargas512
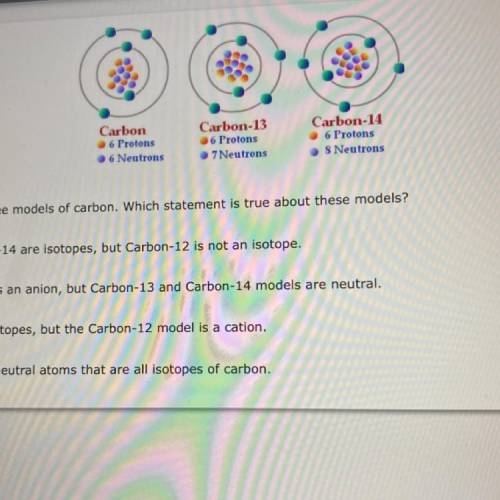
The diagram above shows three models of carbon. Which statement is true about these models?
(DOK 2)
A. Carbon-13 and Carbon-14 are isotopes, but Carbon-12 is not an isotope.
B. The Carbon-12 model is an anion, but Carbon-13 and Carbon-14 models are neutral.
OC. All three models are isotopes, but the Carbon-12 model is a cation.
D. All three models show neutral atoms that are all isotopes of carbon.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 23.06.2019 01:30, oliviacolaizzi
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1

Chemistry, 23.06.2019 03:50, arimarieestrada
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3
Do you know the correct answer?
The diagram above shows three models of carbon. Which statement is true about these models?
(DOK 2)...
Questions in other subjects:

Social Studies, 22.09.2021 06:10

Mathematics, 22.09.2021 06:10

Mathematics, 22.09.2021 06:10

Mathematics, 22.09.2021 06:10











