
Chemistry, 18.02.2021 09:20, kaylamount
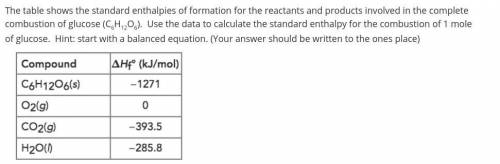
The table shows the standard enthalpies of formation for the reactants and products involved in the complete combustion of glucose (C6H12O6). Use the data to calculate the standard enthalpy for the combustion of 1 mole of glucose. Hint: start with a balanced equation. (Your answer should be written to the ones place)

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:40, yah2muchh
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 07:40, caleb19moody
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 13:10, kellinvagneur
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1
Do you know the correct answer?
The table shows the standard enthalpies of formation for the reactants and products involved in the...
Questions in other subjects:

Spanish, 20.04.2021 19:20



Mathematics, 20.04.2021 19:20


Mathematics, 20.04.2021 19:20


English, 20.04.2021 19:20


Chemistry, 20.04.2021 19:20






