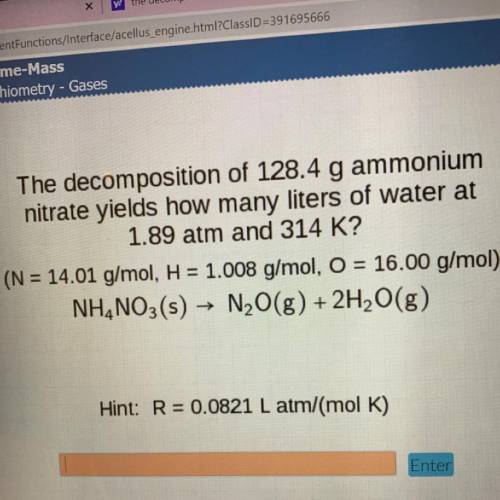
The decomposition of 128.4 g ammonium
nitrate yields how many liters of water at
1.89 atm and...


Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:30, adrian08022
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 08:00, tchase0616
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1
Do you know the correct answer?
Questions in other subjects:






Social Studies, 15.12.2020 03:10

Social Studies, 15.12.2020 03:10

History, 15.12.2020 03:10

Chemistry, 15.12.2020 03:10