
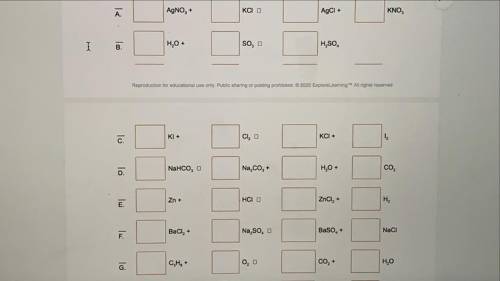
Balance each of the chemical equations below. (Some equations may already be in balance.) In the space above of the letters (A, B,C...), classify the reaction as a synthesis (S), decomposition (D), single replacement (SR), or double replacement (DR) reaction i will give brainliest :)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:20, sindy35111
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l. s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3


Chemistry, 23.06.2019 01:00, kaykardash
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2
Do you know the correct answer?
Balance each of the chemical equations below. (Some equations may already be in balance.) In the spa...
Questions in other subjects:





Mathematics, 14.04.2020 15:54










