
Chemistry, 18.02.2021 01:00, burritomadness
How do I make the molar masses for 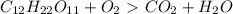 (reactant and products) equal? Please, tell me the steps and how to recalculate.
(reactant and products) equal? Please, tell me the steps and how to recalculate.

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:30, villarrealc1987
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Do you know the correct answer?
How do I make the molar masses for (reactant and products) equal? Please, tell me the steps and how...
Questions in other subjects:

Mathematics, 10.01.2020 09:31



English, 10.01.2020 09:31












