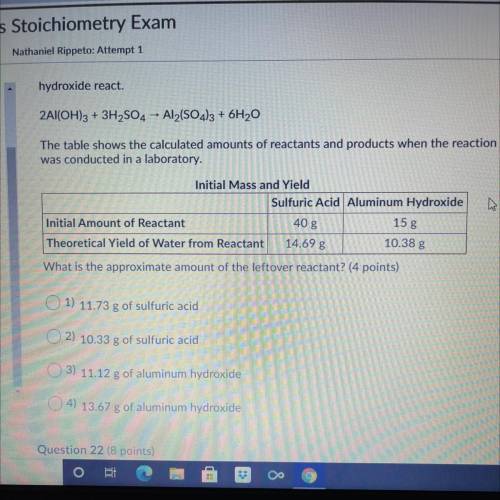
hydroxide react.


Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, jwood287375
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 18:10, sangamlama
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1


Chemistry, 23.06.2019 06:10, jamesgotqui6
2. what two items do autotrophs take from the environment to produce their food? 3. what are the two items that are released during transpiration from leaves? 4. what are the two membranes of the system? a. what are the two stages of photosynthesis? what are the two parts of photosynthesis?
Answers: 2
Do you know the correct answer?
The following reaction shows the products when sulfuric acid and aluminum
hydroxide react.
hydroxide react.
Questions in other subjects:



History, 10.03.2020 19:53

Physics, 10.03.2020 19:53

English, 10.03.2020 19:53

Biology, 10.03.2020 19:53


Mathematics, 10.03.2020 19:54


English, 10.03.2020 19:54