
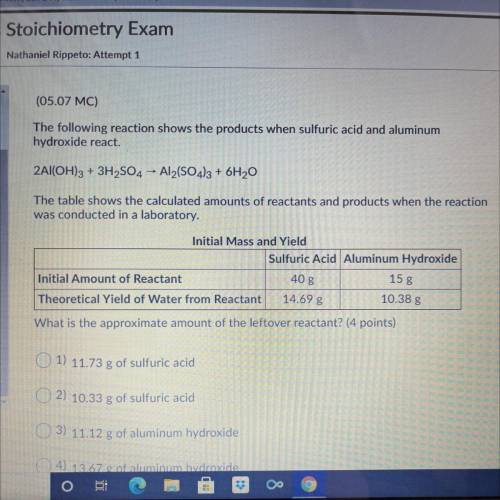
The following reaction shows the products when sulfuric acid and aluminum
hydroxide react.
2Al(OH)3 + 3H2SO4 - Al2(SO4)3 + 6H20
The table shows the calculated amounts of reactants and products when the reaction
was conducted in a laboratory.
What is the approximate amount of the leftover reactant?

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 21:30, sierradanielle9280
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 23.06.2019 03:30, LlayahHarbin
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1
Do you know the correct answer?
The following reaction shows the products when sulfuric acid and aluminum
hydroxide react.
2A...
2A...
Questions in other subjects:

Social Studies, 18.07.2019 20:00





English, 18.07.2019 20:00


History, 18.07.2019 20:00


English, 18.07.2019 20:00






