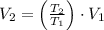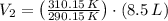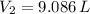Chemistry, 17.02.2021 20:40, Mypasswordishotdog11
Nitrogen was heated from 17°C to 37°C. The original volume of nitrogen was 8.5 L. Find the new volume in liters assuming P and n remain the same.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 20:00, denaemarie02
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 23.06.2019 02:00, bagofmud8339
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1
Do you know the correct answer?
Nitrogen was heated from 17°C to 37°C. The original volume of nitrogen was 8.5 L. Find the new volum...
Questions in other subjects:


Social Studies, 04.08.2019 00:50



Business, 04.08.2019 00:50





Biology, 04.08.2019 00:50


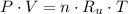 (1)
(1)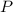 - Pressure, measured in atmospheres.
- Pressure, measured in atmospheres.  - Volumen, measured in liters.
- Volumen, measured in liters.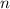 - Molar amount, measured in moles.
- Molar amount, measured in moles. 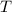 - Temperature, measured in Kelvin.
- Temperature, measured in Kelvin.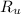 - Ideal gas constant, measured in atmosphere-liters per mole-Kelvin.
- Ideal gas constant, measured in atmosphere-liters per mole-Kelvin.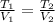 (2)
(2)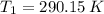 ,
, 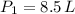 and
and 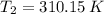 , then the new volume of the gas is:
, then the new volume of the gas is: