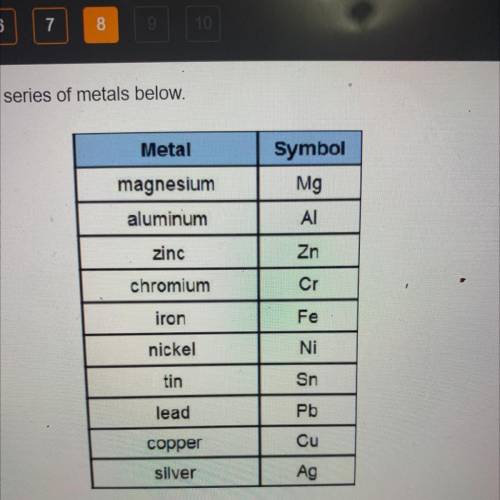
The following reaction occurs in an electrochemical cell.
Sn2++ Pb → Pb2++ Sn
What type of el...


Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:40, bananaslada
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1



Chemistry, 23.06.2019 03:00, cabreradesirae4807
Select the correct answer. wax is a nonpolar substance. in which type of substance is it most soluble?
Answers: 2
Do you know the correct answer?
Questions in other subjects:

English, 10.09.2021 20:50

Mathematics, 10.09.2021 20:50



Advanced Placement (AP), 10.09.2021 21:00

Biology, 10.09.2021 21:00


Health, 10.09.2021 21:00


Mathematics, 10.09.2021 21:00