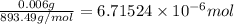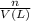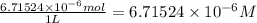Chemistry, 14.10.2019 05:30, skylerdemi1
So my chem experiment asks us: what is the molarity of a 6.0 ppm solution (it's a 6.0 ppm chlorophyll solution, hexane is the solvent)
now, i've found a few ways to solve for it but they both give me different values. if it's possible, could you just tell me which is right.
#1 way: 6parts/1million --> 0.006g/l --> take 0.006g convert w/ molar mass chlorophyll (1 mole/ 893.49 g) --> 6.71524 *10^(-6) moles
put 6.71524 *10^(-6) over 1l --> 6.71524 *10^(-6) m
0r 6parts/1million --> 6g/1000,000g --> take 6g convert w/ molar mass chlorophyll (1 mole/ 893.49 g) --> 6.71524 *10^(-3) moles
use hexane's density to convert 1000,000g to (ml first then) l (density: 1ml/0.6548g) --> 1527.18388 l
put 6.71524 *10^(-3) moles over 1527.18388 l --> 4.397*10^(-6) m
or 6parts/1million --> 6g/1000,000g take 6g convert w/ molar mass chlorophyll (1 mole/ 893.49 g) --> 6.71524 *10^(-3) moles
put 6.71524 *10^(-3) moles over 1000,000 --> 6.71524 *10^(-9) m
i think the second one is the right one but i'm not sure. could you me? !

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, xoxokaydavis5837
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 02:00, webbhlharryteach
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1


Chemistry, 22.06.2019 19:30, Adrian12313
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1
Do you know the correct answer?
So my chem experiment asks us: what is the molarity of a 6.0 ppm solution (it's a 6.0 ppm chlorophy...
Questions in other subjects:



Biology, 09.10.2019 04:00

Chemistry, 09.10.2019 04:00


Mathematics, 09.10.2019 04:00




Mathematics, 09.10.2019 04:00