
Chemistry, 17.02.2021 06:20, mohammadhosseinkarim
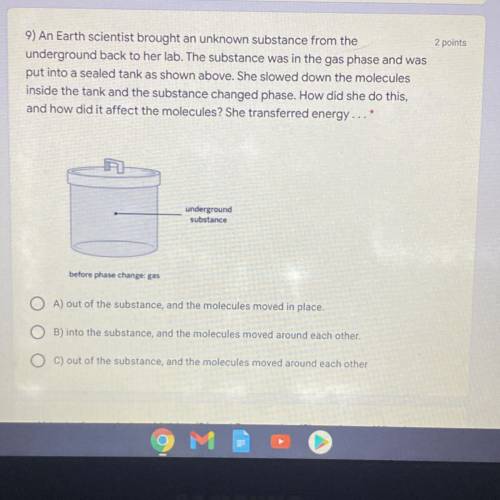
9) An Earth scientist brought an unknown substance from theunderground back to her lab. The substance was in the gas phase and was
put into a sealed tank as shown above. She slowed down the molecules
inside the tank and the substance changed phase. How did she do this,
and how did it affect the molecules? She transferred energy...*

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, penny3109
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1


Do you know the correct answer?
9) An Earth scientist brought an unknown substance from theunderground back to her lab. The substanc...
Questions in other subjects:

Biology, 15.12.2020 23:20

Arts, 15.12.2020 23:20



Business, 15.12.2020 23:20

Mathematics, 15.12.2020 23:20




Mathematics, 15.12.2020 23:20






