
Chemistry, 16.02.2021 04:40, cooltrey777
The first step in the reaction of Alka–Seltzer with stomach acid consists of one mole of sodium bicarbonate (NaHCO3) reacting with one mole of hydrochloric acid (HCl) to produce one mole of carbonic acid (H2CO3), and one mole of sodium chloride (NaCl). Using this chemical stoichiometry, determine the number of moles of carbonic acid that can be produced from 5 mol NaHCO3 and 9 mol HCl.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:30, megaaan214p61pb7
Which compounds have the empirical formula ch2o? a. c2h4o2 b. c3h6o3 c. ch2o2 d. c5h10o5 e. c6h12o6
Answers: 3

Chemistry, 22.06.2019 19:10, asdfghhk9805
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 22.06.2019 22:30, SavageKidKobe
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1
Do you know the correct answer?
The first step in the reaction of Alka–Seltzer with stomach acid consists of one mole of sodium bica...
Questions in other subjects:

Mathematics, 15.05.2021 01:10



Biology, 15.05.2021 01:10


Chemistry, 15.05.2021 01:10



Mathematics, 15.05.2021 01:10


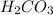 can be produced from 5 mol NaHCO3 and 9 mol HCl.
can be produced from 5 mol NaHCO3 and 9 mol HCl.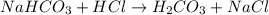
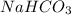 use 1 mole of
use 1 mole of 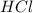
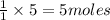 of
of 



