Chemistry, 16.02.2021 04:20, baileyflemingde
Automotive airbags inflate when sodium azide decomposes explosively to its constituent elements. How many grams of sodium azide are required to produce 24.4 L of nitrogen gas at standard temperature and pressure? 2NaN3 --> 2Na + 3N2
47.2 g of sodium azide
106.2 g of sodium azide
1.63 g of sodium azide
0.726 g of sodium azide

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, paynedeforest2596
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1


Chemistry, 22.06.2019 14:30, darkghostmist
What type of reaction fuels the processes seen here?
Answers: 2
Do you know the correct answer?
Automotive airbags inflate when sodium azide decomposes explosively to its constituent elements. How...
Questions in other subjects:

History, 23.08.2021 20:40




Mathematics, 23.08.2021 20:40



English, 23.08.2021 20:40

History, 23.08.2021 20:40


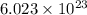 of particles.
of particles.
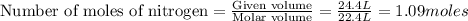
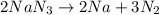
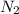 are produced by = 2 moles of
are produced by = 2 moles of 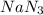
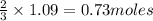 of
of