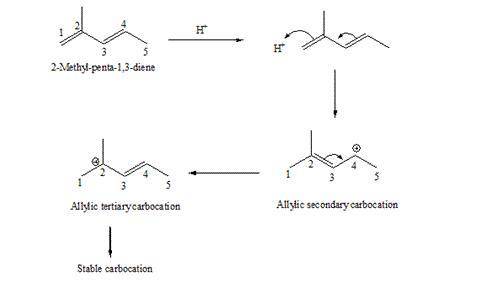
. Write the two resonance hybrids for the carbocation that would be formed by protonation at C-1 of 2-methyl-1,3-pentadiene. Without doing a calculation, would you expect C-2 or C-4 (the two end carbons of the allylic cation) to have the most positive charge on it

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, bobbycisar1205
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 04:40, deedee363
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 12:30, nekathadon
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1
Do you know the correct answer?
. Write the two resonance hybrids for the carbocation that would be formed by protonation at C-1 of...
Questions in other subjects:






Mathematics, 01.07.2021 01:20

History, 01.07.2021 01:20



Mathematics, 01.07.2021 01:20