
Chemistry, 15.02.2021 22:10, marandahuber
N2+3H2=>>2NH3. If 6.72dm³ of each of Nitrogen and hydrogen (measured at S. T.P.) are made to produced Ammonium. Which of the reacted will be in excess and by how many moles?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:40, vpowell5371
Astudent made the lewis dot diagram of a compound shown. what is the error in the lewis dot diagram? a)an o atom should transfer all of its six electrons to mg because the formula is mgo b) both electrons of mg should be transferred to one o adam because the formula is mgo c) the electrons should be transferred from each o add him to capital mg because mg has fewer electrons d) the number of dots around mg should be four because it has to transfer two electrons to each o
Answers: 1

Chemistry, 21.06.2019 22:30, tot92
Imagine that you’re getting ready to move to a new city. when people move, they are influenced by push factors and pull factors, and you have many reasons for your move. which of the following factors is an example of a pull factor? a. wanting to move because you’ve found a great new school somewhere new b. needing to move because there are not enough resources in your old hometown c. being forced to move because your old home is gone d. having to move because there are no jobs in your current hometown
Answers: 1

Chemistry, 22.06.2019 01:30, alfarodougoy8lvt
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 18:20, juansebas35
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
Do you know the correct answer?
N2+3H2=>>2NH3. If 6.72dm³ of each of Nitrogen and hydrogen (measured at S. T.P.) are made to p...
Questions in other subjects:


English, 25.01.2021 21:10

Mathematics, 25.01.2021 21:10


Mathematics, 25.01.2021 21:10


Biology, 25.01.2021 21:10



Social Studies, 25.01.2021 21:10


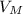 = 22, 4 l/mol
= 22, 4 l/mol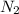 ) = 6,72
) = 6,72 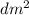 = 6,72 l
= 6,72 l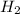 ) = 6,72
) = 6,72 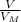
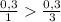 (we have more hydrogen than is need for the reaction)
(we have more hydrogen than is need for the reaction)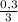 = 0,1 mol (
= 0,1 mol (



