Chemistry, 15.02.2021 20:20, princessroyal
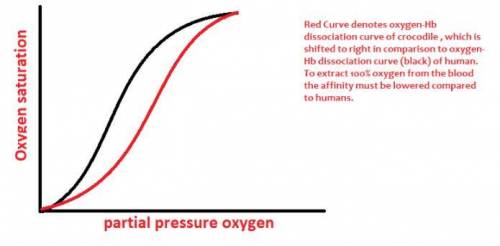
The crocodile which can remain underwater without breathing for up to an hour, drowns it air-breathing prey and then dines on them at its leisure. An adaptation that aids the crocodile in doing so is that it can utilize virtually 100% of the O2 in its blood whereas humans, for example, can extract only -65% of the O2 in their blood. (a) Draw an O2 binding curve comparing crocodile hemoglobin to that of normal hemoglobin. humne ih (b) Experiments have demonstrated that 2,3 bis-phosphoglycerate has no effect on the O2 binding of crocodile hemoglobin. However, it has been shown that 2 molecules of bicarbonate bind to deoxyhemoglobin tetramers but not to oxyhemoglobin tetramers of crocodile hemoglobin. Describe how bicarbonate might effect the O2 affinity of crocodile hemoglobin. Make sure to detail what regions of the hemoglobin tetramek might be bound by bicarbonate and how this would influence structural changes in the crocodile hemoglobin that would, in turn, affect O2 affinity. (c) Sketch the biochemical reactions in red blood cells that result in bicarbonate formation in venous blood. Write balanced equations and identify any enzymes that might be involved. If an enzyme is required, indicate which reaction is enzyme-catalyzed.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, maddyjones4172
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 05:40, Maryjasmine8001
Salicylic acid is a very important acid. it is used to synthesize the aspirin by treating with acetic anhydride. a 0.2015-g sample of salicylic acid was dissolved in a 100.00-ml volumetric flask, and the solution was diluted to the mark. a 10-ml aliquot of this solution was titrated with standard naoh (0.01130 + 0.2% n) to a phenolphthalein faint pink color end point at 19.81 ml. (a) (calculate the normality of the salicylic acid solution used in the titration. (b) assuming the salicylic acid is pure, what is the equivalent weight of the salicylic acid? practice problems for the final exam (continued) (c) (calculate the inherent error in the determination of the equivalent weight you calculated in part (b). use the following absolute errors in the equipment /glassware when calculating the inherent error. 5.00-ml pipet: + 0.02 ml 100-ml volumetric flask: + 0.08 ml analytical balance: + 0.2 mg 25-ml buret: + 0.03 ml
Answers: 2

Chemistry, 22.06.2019 11:20, ashiteru123
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 23.06.2019 06:20, Lindsay882
Type the correct answer in each box. balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1
Do you know the correct answer?
The crocodile which can remain underwater without breathing for up to an hour, drowns it air-breathi...
Questions in other subjects:


Social Studies, 24.12.2019 20:31


French, 24.12.2019 20:31


History, 24.12.2019 20:31

Mathematics, 24.12.2019 20:31

History, 24.12.2019 20:31


Mathematics, 24.12.2019 20:31