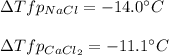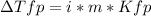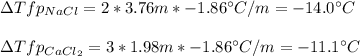Chemistry, 15.02.2021 20:10, battlemarshmell
Sodium chloride (NaCl) is commonly used to melt ice on roads during the winter. Calcium chloride (CaCl2) is sometimes used for this purpose too. Let us compare the effectiveness of equal masses of these two compounds in lowering the freezing point of water by calculating the freezing point depression of solutions containing 220. g of each salt in 1.00 kg of water. (An advantage of is that it acts more quickly because it is hygroscopic, that is, it absorbs moisture from the air to create a solution and begin the process. A disadvantage is that this compound is more costly.) Assume full dissociation of ionic compounds. Kfp(H2O)= -1.86 °C/m.
ΔTfp= °C for NaCl
ΔTfp= °C for CaCl2

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, jordan5778
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 03:30, jabper5522
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1


Chemistry, 22.06.2019 17:00, princessakosua2
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
Do you know the correct answer?
Sodium chloride (NaCl) is commonly used to melt ice on roads during the winter. Calcium chloride (Ca...
Questions in other subjects:


English, 25.07.2019 08:00




Mathematics, 25.07.2019 08:00



Social Studies, 25.07.2019 08:00