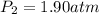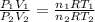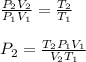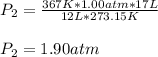Chemistry, 15.02.2021 19:00, noneofurbznessp2yc11
You have 17 liters of gas at STP. If the temperature rises to 94C and while the volume decreases to 12 liters, what will the new pressure be?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 20:20, mgavyn1052
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Do you know the correct answer?
You have 17 liters of gas at STP. If the temperature rises to 94C and while the volume decreases to...
Questions in other subjects:

Physics, 16.01.2022 09:40




Mathematics, 16.01.2022 09:40



Mathematics, 16.01.2022 09:40

Chemistry, 16.01.2022 09:40