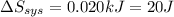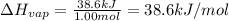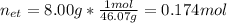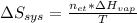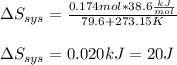Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, salvadorperez26
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 09:30, lisbet123085
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Do you know the correct answer?
It takes 38.6 kJ of energy to vaporize 1.00 mol of ethanol (MW: 46.07 g/mol). What will be ΔSsys for...
Questions in other subjects:

Mathematics, 09.02.2021 01:00


Mathematics, 09.02.2021 01:00




Geography, 09.02.2021 01:00

Arts, 09.02.2021 01:00

Mathematics, 09.02.2021 01:00