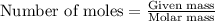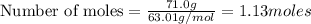Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:30, Sumitco9578
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 22.06.2019 22:30, lanashanabJHsbd1099
Who discovered a pattern to the elements in 1869?
Answers: 1

Chemistry, 23.06.2019 00:00, savyblue1724707
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3
Do you know the correct answer?
A nitric acid solution containing 71.0% HNO3 (by mass) is available in a laboratory. How many moles...
Questions in other subjects:

Mathematics, 18.10.2019 22:30

Computers and Technology, 18.10.2019 22:30

Mathematics, 18.10.2019 22:30


Mathematics, 18.10.2019 22:30

World Languages, 18.10.2019 22:30



History, 18.10.2019 22:30


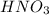 means 71.0 g of
means 71.0 g of