
Chemistry, 12.02.2021 19:30, edgartorres5123
Which is true about the compounds carbon monoxide (CO) and carbon dioxide (CO2)?
A. The compounds have the same properties because they contain the same elements.
B. The compounds have different properties because they are found in different amounts in the atmosphere.
C. The compounds have the same properties because they are both gases.
D. The compounds have different properties because they contain the same elements in different quantities.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:10, amuijakobp78deg
Agas mixture with a total pressure of 745 mmhg contains each of the following gases at the indicated partial pressures: co2, 245 mmhg ; ar, 119 mmhg ; and o2, 163 mmhg . the mixture also contains helium gas. part a what is the partial pressure of the helium gas? phe p h e = nothing mmhg request answer part b what mass of helium gas is present in a 10.2-l sample of this mixture at 283 k ? m m = nothing g request answer
Answers: 1


Chemistry, 22.06.2019 08:00, hdjsjfjruejchhehd
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 14:30, srutkowske1489
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1
Do you know the correct answer?
Which is true about the compounds carbon monoxide (CO) and carbon dioxide (CO2)?
A. The compounds h...
Questions in other subjects:

Mathematics, 31.03.2021 18:00

Business, 31.03.2021 18:00



English, 31.03.2021 18:00


English, 31.03.2021 18:00

English, 31.03.2021 18:00

English, 31.03.2021 18:00

Mathematics, 31.03.2021 18:00


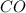 contains carbon and oxygen in the ratio of 3: 4 by mass whereas
contains carbon and oxygen in the ratio of 3: 4 by mass whereas 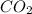 contains carbon and oxygen in the ratio of 3: 8 by mass.
contains carbon and oxygen in the ratio of 3: 8 by mass.



