
Chemistry, 28.01.2020 15:56, vallhernandez13
The wavelength of light is 310. nm; calculate the frequency.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:50, josie311251
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3


Chemistry, 23.06.2019 02:00, raulflores01
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
Do you know the correct answer?
The wavelength of light is 310. nm; calculate the frequency....
Questions in other subjects:

Chemistry, 12.07.2019 22:30


Social Studies, 12.07.2019 22:30

Social Studies, 12.07.2019 22:30

Mathematics, 12.07.2019 22:30


Mathematics, 12.07.2019 22:30

Mathematics, 12.07.2019 22:30

Geography, 12.07.2019 22:30

English, 12.07.2019 22:30


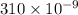 m. Relation between wavelength, speed of light and frequency is as follows.
m. Relation between wavelength, speed of light and frequency is as follows.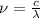
 = frequency
= frequency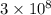 m/s
m/s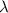 = wavelength
= wavelength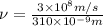
 per second
per second



