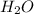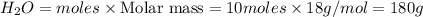Chemistry, 12.02.2021 16:20, queen200760
Calculate the mass of water produced from the reaction of 24.0 g of H2 and 160.0 g of O2. What is the limiting reagent?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:20, greenbyron88
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 21:00, nsutton9985
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1

Chemistry, 23.06.2019 06:30, tdahna0403
The molar mass of cu is 63.55 g/mol. the number of grams of cu produced in this reaction is
Answers: 3
Do you know the correct answer?
Calculate the mass of water produced from the reaction of 24.0 g of H2 and 160.0 g of O2. What is th...
Questions in other subjects:

Chemistry, 11.03.2021 15:40

Spanish, 11.03.2021 15:40



Mathematics, 11.03.2021 15:40

Mathematics, 11.03.2021 15:40


Spanish, 11.03.2021 15:40

Arts, 11.03.2021 15:40


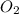 is the limiting reagent, 180 g of water is produced
is the limiting reagent, 180 g of water is produced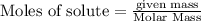
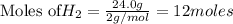
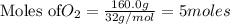
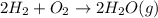
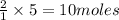 of
of