
Chemistry, 19.09.2019 18:30, woodfordmaliky
Consider these reactions where m represents a generic metal
1. 2m(s) +6hcl(aq) --> 2mcl3(aq)+3h2(g) (deltah)= -725.0 kj
2. hcl(> hcl(aq) (deltah)= -74.8kj 3. h2(g)+cl2(g) --> 2hcl(g) (deltah)=-1845.0kj 4. mcl3(s) --> mcl3(aq) (deltah)= -476.0kj use the information above to determine the enthalpy of the following reaction.
2m(s)+3cl2(g) > 2mcl3(s) (deltah) = kj

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:10, alvaradolm6853
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 14:00, JJlover1892
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2
Do you know the correct answer?
Consider these reactions where m represents a generic metal
1. 2m(s) +6hcl(aq) --> 2mcl3(a...
1. 2m(s) +6hcl(aq) --> 2mcl3(a...
Questions in other subjects:


Mathematics, 12.12.2019 20:31

History, 12.12.2019 20:31

Mathematics, 12.12.2019 20:31

History, 12.12.2019 20:31



Physics, 12.12.2019 20:31


Chemistry, 12.12.2019 20:31


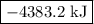
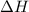 of an overall reaction is obtained by adding the enthalpy change for each individual step reaction involved to obtain the overall reaction.
of an overall reaction is obtained by adding the enthalpy change for each individual step reaction involved to obtain the overall reaction.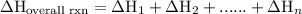
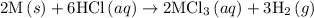 ...… (1)
...… (1)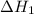 is -725 kJ.
is -725 kJ.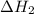 .
.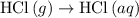 ...... (2)
...... (2) 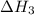 .
.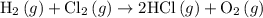 …… (3)
…… (3)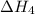 .
.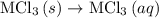 …… (4)
…… (4)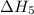 .
.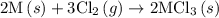 …… (5)
…… (5)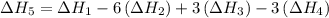 ...... (7)
...... (7) 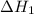 , -74.8 kJ for
, -74.8 kJ for 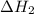 and -1845 kJ for
and -1845 kJ for 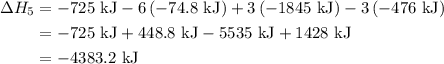
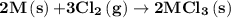 is -4383.2 kJ.
is -4383.2 kJ.



