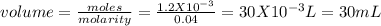Chemistry, 04.02.2020 12:58, abhibhambhani
20.0 ml of 0.06 m hcl (in a flask) is titrated with 0.04 m naoh (in a burette). how many milliliters of naoh needs to be used to reach the equivalence point?
a.0.03 ml
b.20.0 ml
c.20 ml
d.30 ml
2.which of the following would you identify a titration curve that involved a strong acid titrated by a weak base?
a. the ph at the equivalence point is lower than 7.
b. the ph at the equivalence point is higher than 7.
c. the titration curve begins at a higher ph and ends at a lower ph.
d. there is a rapid change in ph near the equivalence point (ph = 7).

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:00, chefdnguyen
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 23.06.2019 13:30, MalikaJones
Determine the rate law, including the values of the orders and rate law constant, for the following reaction using the experimental data provided. a + b yields products trial [a] [b] rate 1 0.30 m 0.25 m 1.2 × 10-2 m/min 2 0.30 m 0.50 m 4.8 × 10-2 m/min 3 0.60 m 0.50 m 9.6 × 10-2 m/min
Answers: 1

Chemistry, 23.06.2019 21:00, marivi3cazares
Examine these two msds from different manufacturers for sodium hydroxide (naoh). compare and contrast the following aspects: chemical names, chemical properties, order of components, health hazards, and proper disposal. click on each of the links to examine two msds reports from different sources. sodium hydroxide version 1 sodium hydroxide version 2
Answers: 1
Do you know the correct answer?
20.0 ml of 0.06 m hcl (in a flask) is titrated with 0.04 m naoh (in a burette). how many milliliters...
Questions in other subjects:



English, 18.01.2020 01:31

Mathematics, 18.01.2020 01:31