Chemistry, 11.02.2021 22:20, angelinagiraffp538zb
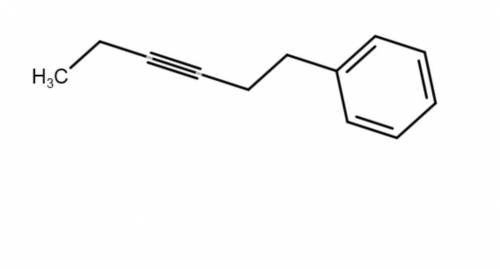
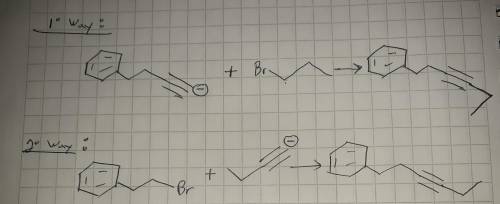
This molecule can be synthesized from an alkyne anion and an alkyl bromide. However, there are two ways in which this molecule can be formed. One way uses a higher molecular weight alkyne anion (Part 1) and the other uses a lower molecular weight anion (Part 2). Draw the two versions in the boxes below. Omit spectator ions.
For Part 1: Draw the reactants (i. e., alkyne anion and alkyl bromide) needed for the pathway that uses a higher molecular weight alkyne anion:
For Part 2: Draw the reactants (i. e., alkyne anion and alkyl bromide) needed for the pathway that uses a lower molecular weight alkyne anion:

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:30, janetexcoelho
What does the mass of 0.7891 mol of ferric oxide (fe2o3)
Answers: 1

Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 10:40, trinityanne1738
Asolid that forms and separates from a liquid mixture is called
Answers: 2
Do you know the correct answer?
This molecule can be synthesized from an alkyne anion and an alkyl bromide. However, there are two w...
Questions in other subjects:










Chemistry, 24.07.2019 23:10