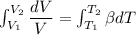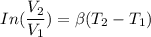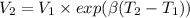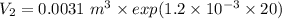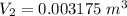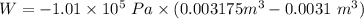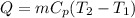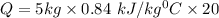Chemistry, 11.02.2021 21:20, st23pgardner
Five kilograms of liquid carbon tetrachloride undergo a mechanically reversible, isobaric change of state at 1 bar during which the temperature changes from 0∘C to 20∘C0 ∘ C to 20 ∘ C Determine ΔVt, W,Q,ΔHt, and ΔUt.ΔV t ,W, Q,ΔH t , and ΔU t . The properties for liquid carbon tetrachloride at 1 bar and 0∘C0 ∘ C may be assumed independent of temperature: β=1.2×10−3K−1,CP=0.84kJ⋅kg−1⋅K−1, and rho=1590kg⋅m−3β=1.2×10 −3 K −1 ,C P =0.84kJ⋅kg −1 ⋅K −1 , and rho=1590kg⋅m −3

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, codeyhatch142
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1

Chemistry, 22.06.2019 10:00, alexabdercmur
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 23.06.2019 01:00, davelopez979
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1

Chemistry, 23.06.2019 10:30, GiuliAzevedo
The element chlorine has two stable isotopes, chlorine-35 with a mass of 34.97 amu and chlorine-37 with a mass of 36.95 amu. from the atomic weight of cl = 35.45 one can conclude that:
Answers: 2
Do you know the correct answer?
Five kilograms of liquid carbon tetrachloride undergo a mechanically reversible, isobaric change of...
Questions in other subjects:

Mathematics, 03.11.2020 23:20

History, 03.11.2020 23:20





Business, 03.11.2020 23:20

Social Studies, 03.11.2020 23:20


Mathematics, 03.11.2020 23:20


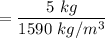
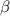 is independent of temperature while pressure is constant;
is independent of temperature while pressure is constant;