
Chemistry, 11.02.2021 14:00, moomoofower
How many moles of silver would precipitate out when 3.6 x 10^23 atoms of zinc are combined in silver nitrate according to the following unbalanced equation? (Balance equation first!)
Zn + AgNO3 → Zn(NO3)2 +Ag
A: 1.67 moles
B: 1.2 moles
C: 3.34 moles
D: 0.6 moles
Can someone please help me with this I don’t get it at all.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, cadenhuggins2
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1



Chemistry, 23.06.2019 06:00, BigGirlsTheBest
Amanda pushes a box across the room with a force of 30 n. it accelerates at 5 m/s/s. what is the mass of the box? * 6 kg 1.16 kg 30 kg 5kg
Answers: 2
Do you know the correct answer?
How many moles of silver would precipitate out when 3.6 x 10^23 atoms of zinc are combined in silver...
Questions in other subjects:

Mathematics, 11.03.2020 06:32


Social Studies, 11.03.2020 06:32

Mathematics, 11.03.2020 06:32


Mathematics, 11.03.2020 06:32





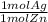 = 0.6 mol Ag
= 0.6 mol Ag




