
The reaction between hydrogen and oxygen to yield water vapor has δh∘=−484kj: 2h2(g)+o2(g)→2h2o(g)δh∘=−484kj
how much pv work is done in kilojoules for the reaction of 3.20 mol of h2 with 1.60 mol of o2 at atmospheric pressure if the volume change is −22.6l?
express your answer using three significant figures

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 18:50, cj31150631
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 23.06.2019 00:00, familyk0jj3
How do you determine the percent yield of a chemical reaction
Answers: 1
Do you know the correct answer?
The reaction between hydrogen and oxygen to yield water vapor has δh∘=−484kj: 2h2(g)+o2(g)→2h2o(g)δ...
Questions in other subjects:




Mathematics, 10.06.2021 16:50




Mathematics, 10.06.2021 16:50



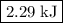 .
.
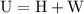
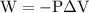
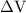 is the change in the volume in liter.
is the change in the volume in liter.
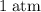 .
.
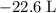 .
.
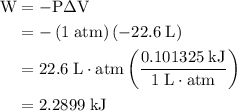
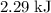 .
.




