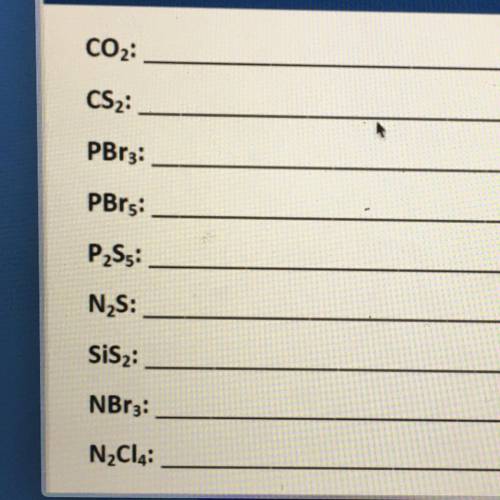
Name the binary covalent compounds.
CO2:
CS2:
PBr3:
P2S5:
N2S:
SiS2:<...


Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, cynthiagutierrez65
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1

Chemistry, 21.06.2019 18:30, jadepotts3965
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2


Chemistry, 22.06.2019 15:00, levelebeasley1
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2
Do you know the correct answer?
Questions in other subjects:



Physics, 19.03.2021 18:50

Mathematics, 19.03.2021 18:50

Chemistry, 19.03.2021 18:50


Social Studies, 19.03.2021 18:50