
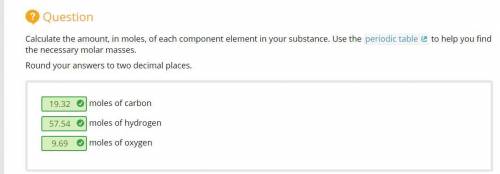
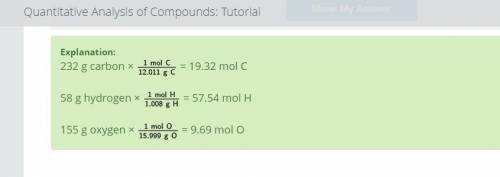
Calculate the amount, in moles, of each component element in your substance. Use the periodic table to help you find the necessary molar masses. Round your answers to two decimal places.
ANSWER:
19.32 moles of carbon
57.54 moles of hydrogen
9.69 moles of oxygen
IK THE ANSWER GUYS. THIS IS JUST FOR THOSE WHO NEEDS HELP WITH THIS QUESTION XXX

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:40, larkinc2946
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1

Chemistry, 23.06.2019 00:30, emilylizbeth12334
Which of the following best describes technology a. something created for only scientists to use b. the method of thinking that scientists use. c. the application of engineering to create useful products. c. a scientific idea
Answers: 1

Chemistry, 23.06.2019 01:00, aliviadushane
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1
Do you know the correct answer?
Calculate the amount, in moles, of each component element in your substance. Use the periodic table...
Questions in other subjects:





Mathematics, 30.04.2021 23:50



Mathematics, 30.04.2021 23:50







