
Chemistry, 10.02.2021 22:20, gracethegreat1
Identify both of the PE wells in the graph below as belonging to either a covalent bond or an LDF. Then, explain what this means regarding the energy needed to break a covalent bond.
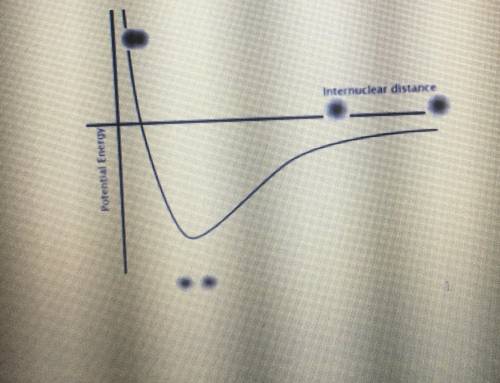

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, kdenormandie3122
Geothermal energy for industrial use is available almost anywhere. a. true b. false
Answers: 2


Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 22.06.2019 19:40, powberier6979
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1
Do you know the correct answer?
Identify both of the PE wells in the graph below as belonging to either a covalent bond or an LDF. T...
Questions in other subjects:


Mathematics, 20.04.2020 23:11




History, 20.04.2020 23:11

Mathematics, 20.04.2020 23:11

History, 20.04.2020 23:11

Mathematics, 20.04.2020 23:11






