Use for questions 1-4
Zn + 2HCl → ZnCl2 + H2
1) If 0.8 moles of Zn are used,
how...

Chemistry, 10.02.2021 20:50, NikolaiSolokov
Use for questions 1-4
Zn + 2HCl → ZnCl2 + H2
1) If 0.8 moles of Zn are used,
how many moles of HCl are
required?
2) If 3.5 moles of Zn are used,
how many moles of HCl are
required?
3) If 4.8 moles of HCl are used,
how many moles of H, are
produced?
4) If 0.48 moles of ZnCl, are
produced, how many moles of
HCl are needed?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:10, maribel2421
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

Chemistry, 22.06.2019 05:40, wanderer3653
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 09:40, kolibeilfuss
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 10:10, jojomgarcia01
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Social Studies, 31.03.2020 20:33


Mathematics, 31.03.2020 20:33


Mathematics, 31.03.2020 20:33


Social Studies, 31.03.2020 20:33

Mathematics, 31.03.2020 20:34


Business, 31.03.2020 20:34


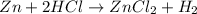
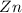 require = 2 moles of
require = 2 moles of 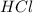
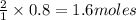 of
of  of
of 
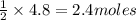 of
of 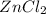 are produced by = 2 moles of
are produced by = 2 moles of  of
of 



