

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 15:30, lizzyhearts
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 21:30, emmalucilleblaha1995
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
Do you know the correct answer?
Student was identifying the formula of 1 mole of unknown powder. When it was placed on a scale, it w...
Questions in other subjects:

Mathematics, 22.05.2021 04:40




Arts, 22.05.2021 04:40






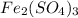
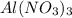 weighs = 26.98(1)+14.01(3)+15.99(9) =212.99 g/mol
weighs = 26.98(1)+14.01(3)+15.99(9) =212.99 g/mol 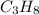 weighs = 12.01(3)+1.007(8) = 44.1 g/mol
weighs = 12.01(3)+1.007(8) = 44.1 g/mol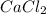 weighs = 40.07(1)+35.5(2)= 110.98 g/mol
weighs = 40.07(1)+35.5(2)= 110.98 g/mol




