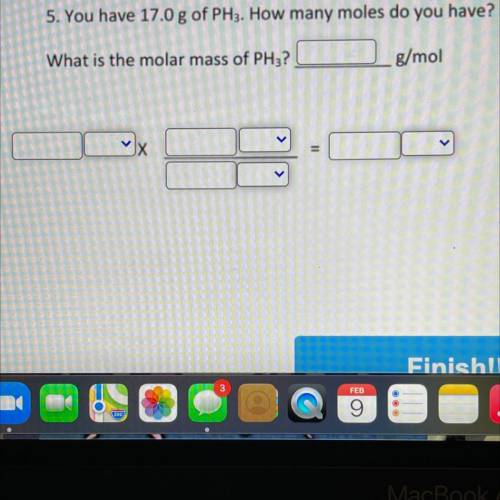
Need helped for this
...

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:30, leenzazou587
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 23.06.2019 01:20, cedricevans41p4j3kx
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 09:00, joelpimentel
The concentration of ionic substances is important for the heart to beat. your heart responds to electrical impulses that travel through heart cells that are made up mostly of water. which properties of ionic compounds are important to support this function? solubility in water conductivity crystalline melting point
Answers: 3

Chemistry, 23.06.2019 16:50, alosnomolina1122
Consider the balanced equation below. pcl3+cl2-> pcl5 what is the mole ratio of pcl3 to pcl5
Answers: 1
Do you know the correct answer?
Questions in other subjects:


History, 22.10.2020 03:01


Mathematics, 22.10.2020 03:01

Mathematics, 22.10.2020 03:01

Mathematics, 22.10.2020 03:01

Mathematics, 22.10.2020 03:01


Chemistry, 22.10.2020 03:01