Consider the equations below
A
CH (9) →C(s)+ 2H2(9) AH= 74,6 kJ
C(s) +2012(g) → CCI,(9)...

Chemistry, 09.02.2021 06:00, rhaquan66766
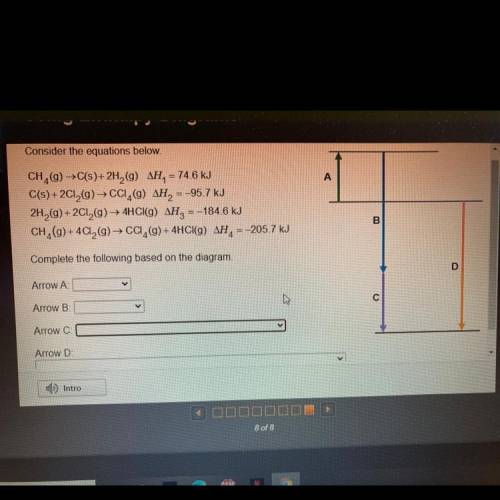
Consider the equations below
A
CH (9) →C(s)+ 2H2(9) AH= 74,6 kJ
C(s) +2012(g) → CCI,(9) AH = -95.7 kJ
2H2(g) +2012(9) — 4HCl(g) AH, =-184.6 kJ
CH_(9)+ 4C12(g) → CC,(g) + 4HCI(g) AH= -205,7 kJ
B
Complete the following based on the diagram
D
Arrow A
✓
Arrow B
no
с
Arrow C
Arrow D

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:20, maevemboucher78
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2

Chemistry, 22.06.2019 15:00, emmalie52
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 17:00, jazmine8194
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 25.07.2019 10:30



History, 25.07.2019 10:30


Social Studies, 25.07.2019 10:30


Health, 25.07.2019 10:30

Geography, 25.07.2019 10:30






