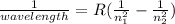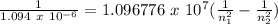Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:30, lizzzzi7908
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1


Chemistry, 23.06.2019 01:50, UncleVictor5188
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1
Do you know the correct answer?
The hydrogen atom can absorb light of wavelength 1094 nm. find the initial and final values of n ass...
Questions in other subjects:


Mathematics, 15.12.2020 08:00

Mathematics, 15.12.2020 08:00


Advanced Placement (AP), 15.12.2020 08:00

Mathematics, 15.12.2020 08:00

Health, 15.12.2020 08:00