40 POINTS!
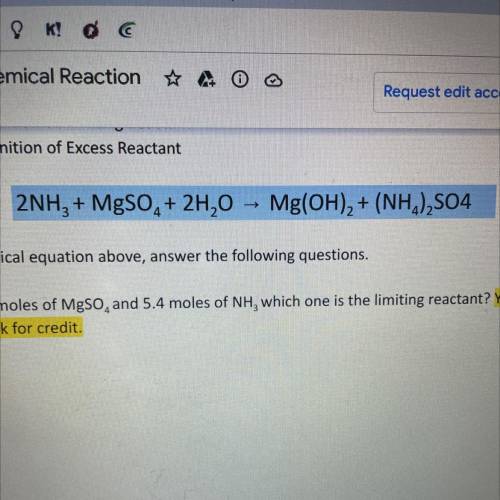
2NH3 + MgSO4 + 2H20 -> Mg(OH)2 + (NH4)2SO4
using the chemical equation ab...

Chemistry, 09.02.2021 01:30, emalvidrez5205
40 POINTS!
2NH3 + MgSO4 + 2H20 -> Mg(OH)2 + (NH4)2SO4
using the chemical equation above answer the following questions. SHOW ALL WORK OR RECEIVE NO CREDIT
A. If I have 4.6 moles of MgSO4 and 5.4 moles of NH3 which one is the limiting reactant? Show work for credit
B. What is the greatest amount of Mg(OH)2 that can be made with 4.6 moles of MgSO4 and 5.4 moles of NH3? Show work for credit
C. How many moles of the excess reactant is left over after the reaction has been completed? Show work for credit

Answers: 1
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 03:00, rhianna18
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 1
Do you know the correct answer?
Questions in other subjects:










Mathematics, 23.06.2019 10:40






