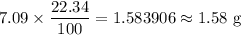Chemistry, 08.02.2021 22:50, amycressey1970
A 10.11 g sample of NaBr contains 22.34 % Na by mass. Considering the law of constant composition (definite proportions), how many grams of sodium does a 7.09 g sample of sodium bromide contain

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 06:00, tddreviews
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 10:00, Cythina2007
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1
Do you know the correct answer?
A 10.11 g sample of NaBr contains 22.34 % Na by mass. Considering the law of constant composition (d...
Questions in other subjects:

Mathematics, 07.10.2020 21:01

Chemistry, 07.10.2020 21:01

Mathematics, 07.10.2020 21:01


Social Studies, 07.10.2020 21:01


Mathematics, 07.10.2020 21:01

Mathematics, 07.10.2020 21:01


Biology, 07.10.2020 21:01


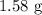
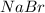 contains 22.34%
contains 22.34% 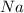 by mass
by mass