Chemistry, 08.02.2021 21:40, sillyvanna
PLEASE HELP ASAP WILL GIVE BRAINLIEST!!
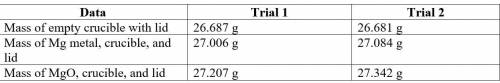
2. Subtract the mass of the crucible and lid (row 1 in the chart) from the total mass of Mg, crucible, and lid (row 2 in the chart) to find the mass of magnesium for each trial.
• Trial 1:
• Trial 2:
3. Subtract the mass of the crucible and lid (row 1 in the chart) from the total mass of MgO, crucible, and lid (row 3 in the chart) to find the mass of magnesium oxide for each trial. This is the actual yield of magnesium oxide for each trial.
• Trial 1:
• Trial 2:
4. Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of MgO for each trial.
• Trial 1:
• Trial 2:
5. Determine the percent yield of MgO for your experiment for each trial.
• Trial 1:
• Trial 2:
6. Determine the average percent yield of MgO for the two trials.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 20:00, Chynadoll94
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 22.06.2019 22:30, robertss403
How many moles of kci are produced from 2.50 moles k
Answers: 1
Do you know the correct answer?
PLEASE HELP ASAP WILL GIVE BRAINLIEST!!
2. Subtract the mass of the crucible and lid (row 1 in the...
Questions in other subjects:

History, 30.06.2019 11:00

Mathematics, 30.06.2019 11:00





Spanish, 30.06.2019 11:00