
Chemistry, 08.02.2021 20:30, SophieStar15
ANSWER ASAP
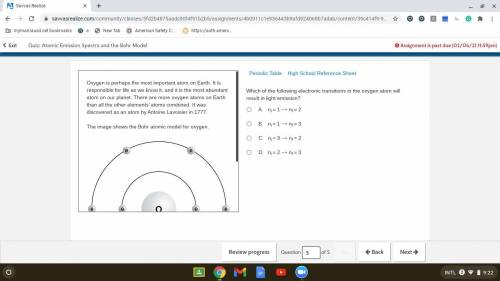
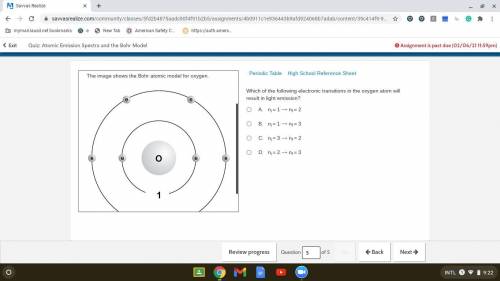
Which of the following electronic transitions in the oxygen atom will result in light emission?
. A. ni = 1 ⟶ nf = 2
B. ni = 1 ⟶ nf = 3
C. ni = 3 ⟶ nf = 2
D. ni = 2 ⟶ nf = 3

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 22:50, kanerobertrosss2213
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3


Chemistry, 23.06.2019 05:00, lifeoflashay1659
He nucleus contains the cells genetic material in the form of dna. dna is organized into our chromosomes, which are made up of thousands of that determine our traits.
Answers: 1
Do you know the correct answer?
ANSWER ASAP
Which of the following electronic transitions in the oxygen atom will result in light e...
Questions in other subjects:



Mathematics, 24.03.2020 14:45




English, 24.03.2020 14:47

Mathematics, 24.03.2020 14:48


Mathematics, 24.03.2020 14:49






