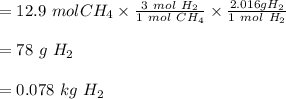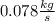The reform reaction between steam and gaseous methane (CH4) produces "synthesis gas," a mixture of carbon monoxide gas and dihydrogen gas. Synthesis gas is one of the most widely used industrial chemicals, and is the major industrial source of hydrogen. Suppose a chemical engineer studying a new catalyst for the reform reaction finds that 924. liters per second of methane are consumed when the reaction is run at 261.°C and 0.96atm. Calculate the rate at which dihydrogen is being produced.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, aubreymoore9441
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 05:00, pandasarecute53
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1


Chemistry, 22.06.2019 14:30, davidrodriguez122001
Which of the following describes a situation where competition between producers exists
Answers: 1
Do you know the correct answer?
The reform reaction between steam and gaseous methane (CH4) produces "synthesis gas," a mixture of c...
Questions in other subjects:

Biology, 25.10.2020 16:00


Social Studies, 25.10.2020 16:00

Mathematics, 25.10.2020 16:00

Mathematics, 25.10.2020 16:00

SAT, 25.10.2020 16:00


Physics, 25.10.2020 16:00

Arts, 25.10.2020 16:00

Arts, 25.10.2020 16:00


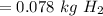 ".
".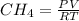


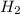 produced:
produced: