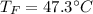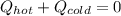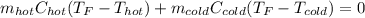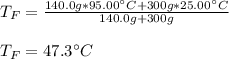Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 06:30, luhmimi17
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 14:00, coylenoah0
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2
Do you know the correct answer?
If you combine 300.0 mL of water at 25.00 ∘C and 140.0 mL of water at 95.00 ∘C, what is the final te...
Questions in other subjects:

English, 18.03.2021 02:10

Social Studies, 18.03.2021 02:10




Mathematics, 18.03.2021 02:10


Social Studies, 18.03.2021 02:10

Mathematics, 18.03.2021 02:10