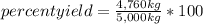A chemical process used to produce ethanol as a fuel additive was expected to produce 5,000 kilograms of ethanol based on the amounts of starting materials used, but only 4,760 kilograms were produced. What was the percent yield for ethanol in this process? A)1.09 percent B)4.80 percent C)95.2 percent D)105 percent

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:00, anferneebcoleman
How many moles of oxygen react with 12 moles of aluminum
Answers: 1

Chemistry, 23.06.2019 05:00, andrwisawesome0
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3


Chemistry, 23.06.2019 14:30, daphnevlogs11
If energy was included in a chemical reaction, on which side of the equation would it be written for an endothermic reaction?
Answers: 1
Do you know the correct answer?
A chemical process used to produce ethanol as a fuel additive was expected to produce 5,000 kilogram...
Questions in other subjects:

English, 14.05.2020 21:57



Mathematics, 14.05.2020 21:57

Mathematics, 14.05.2020 21:57

Social Studies, 14.05.2020 21:57


Mathematics, 14.05.2020 21:57