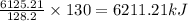Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, shafferakr6
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 03:30, fbillinton
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 19:00, Farhan54019
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 23.06.2019 01:30, Dmoney5104
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2
Do you know the correct answer?
The standard heat of combustion is shown in the following chemical equation CgH 20 (g) + 140 2(g) 9C...
Questions in other subjects:

Mathematics, 06.06.2020 06:58





Business, 06.06.2020 06:58




Mathematics, 06.06.2020 06:58


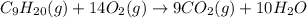
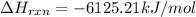 . If 130 g of nonane combusts , how much heat is released?
. If 130 g of nonane combusts , how much heat is released?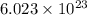 of particles.
of particles.
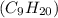 weighs = 128.2 g
weighs = 128.2 g 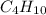 on combustion releases =
on combustion releases =