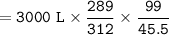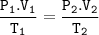Chemistry, 05.02.2021 20:50, miguelturner
If a balloon containing 3000 L of gas at 39°C and 99 kPa rises to an altitude where the pressure is 45.5 kPa and the temperature is 16°C,
the volume of the balloon under these new conditions would be calculated using the following conversion factor ratios:
( the one I have marked, I’m not sure if that’s the answer or not )


Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:00, rodriguezscarlet1713
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2


Do you know the correct answer?
If a balloon containing 3000 L of gas at 39°C and 99 kPa rises to an altitude where the pressure is...
Questions in other subjects:



History, 11.10.2019 22:30




Mathematics, 11.10.2019 22:30


Mathematics, 11.10.2019 22:30