
Chemistry, 05.02.2021 20:10, lovelyheart5337
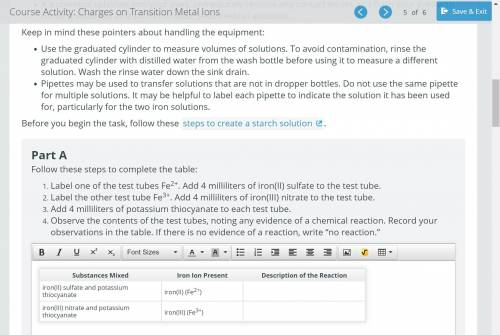
Label one of the test tubes Fe2+. Add 4 milliliters of iron(II) sulfate to the test tube.
Label the other test tube Fe3+. Add 4 milliliters of iron(III) nitrate to the test tube.
Add 4 milliliters of potassium thiocyanate to each test tube.
Observe the contents of the test tubes, noting any evidence of a chemical reaction. Record your observations in the table. If there is no evidence of a reaction, write “no reaction.”

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 09:20, pandaman632
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1
Do you know the correct answer?
Label one of the test tubes Fe2+. Add 4 milliliters of iron(II) sulfate to the test tube.
Label the...
Questions in other subjects:

English, 02.09.2021 21:50

History, 02.09.2021 21:50


Mathematics, 02.09.2021 21:50



English, 02.09.2021 21:50

Mathematics, 02.09.2021 21:50







