
Consider this reaction: 2so2 (g) + o2 (g) 2so3 (g) + heat. what will happen if more sulfur trioxide gas is added to the reaction mixture?
a-the equilibrium will shift toward the products.
b-the equilibrium will shift toward the reactants.
c-keq will increase.
d-keq will decrease.
e-there will be no shift in equilibrium.

Answers: 3
Other questions on the subject: Chemistry



Do you know the correct answer?
Consider this reaction: 2so2 (g) + o2 (g) 2so3 (g) + heat. what will happen if more sulfur trioxide...
Questions in other subjects:






Physics, 23.03.2021 17:40


English, 23.03.2021 17:40

Mathematics, 23.03.2021 17:40


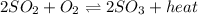
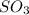 is added
is added



