
Chemistry, 04.02.2021 14:00, bethanybowers4986
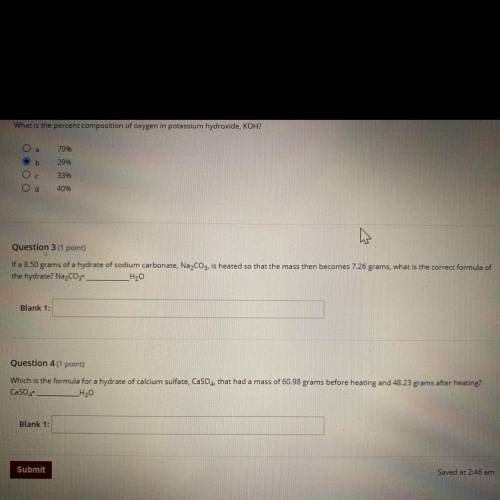
Question 3 (1 point)
If a 8.50 grams of a hydrate of sodium carbonate, Na2CO3, is heated so that the mass then becomes 7.26 grams, what is the correct formula of
the hydrate? Na2CO3 _H20
Blank 1:
Question 4 (1 point)
Which is the formula for a hydrate of calcium sulfate, CaSO4, that had a mass of 60.98 grams before heating and 48.23 grams after heating?
CaSO4
_H20
Blank 1:

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, lucasrandall
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 12:40, jaylen2559
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 14:00, daniel1480
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 21:20, jordan2875
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
Do you know the correct answer?
Question 3 (1 point)
If a 8.50 grams of a hydrate of sodium carbonate, Na2CO3, is heated so that th...
Questions in other subjects:


Social Studies, 02.06.2021 20:20


Mathematics, 02.06.2021 20:20

English, 02.06.2021 20:20

Mathematics, 02.06.2021 20:20









