
Chemistry, 03.02.2021 03:50, lolyourenotpoppunk
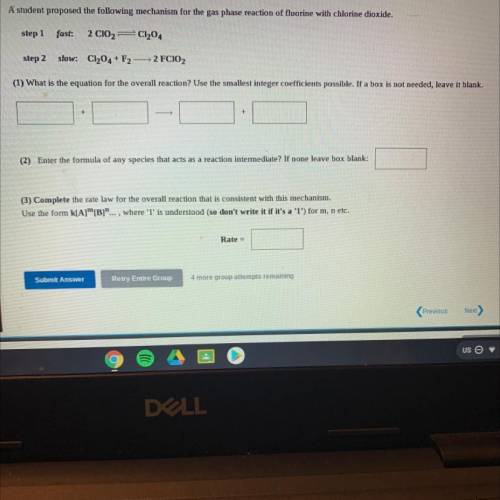
A student proposed the following mechanism for the gas phase reaction of fluorine with chlorine dioxide.
step 1
fast:
2 C102=C1204
step 2
slow: C1204 + F2
2 FCIO2
(1) What is the equation for the overall reaction? Use the smallest integer coefficients possible. If a box is not needed, leave it blank.
+
+
(2) Enter the formula of any species that acts as a reaction intermediate? If none leave box blank:
(3) Complete the rate law for the overall reaction that is consistent with this mechanism.
Use the form k[A][B]"..., where 'l' is understood (so don't write it if it's a '1') for m, n etc.
Rate =

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, nadikadiaz1
These questions are based on the attached photo. the experiment is about burning magnesium metal with oxygen. 1. write the balanced chemical equation for the reaction you are performing. 2. calculate the mass of magnesium metal used in each trial. o trial 1: o trial 2: 3. calculate the actual yield of magnesium oxide for each trial. o trial 1: o trial 2: 4. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. o trial 1: o trial 2: 5. determine the percent yield of mgo for your experiment for each trial. o trial 1: o trial 2: 6. determine the average percent yield of mgo for the two trials. your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. if the average percent yield of this process is higher than that, this could save the company money. what is your recommendation to the company? support your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.
Answers: 1

Chemistry, 22.06.2019 18:00, tatemelliott
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2


Do you know the correct answer?
A student proposed the following mechanism for the gas phase reaction of fluorine with chlorine diox...
Questions in other subjects:

English, 12.08.2020 09:01

English, 12.08.2020 09:01


English, 12.08.2020 09:01



Mathematics, 12.08.2020 09:01


Medicine, 12.08.2020 09:01

Mathematics, 12.08.2020 09:01






